Evidence
Synthesis

What We Do
Let us help you navigate the complexities of market access and deliver your evidence synthesis outputs with confidence and increased payer certainty.
At GIPAM, we are passionate about guiding our clients through the currents of pre- and post-market access challenges with robust, clinically relevant evidence generation. Critical to this journey, is a well-executed evidence synthesis strategy.
Whether you're conducting a targeted literature review (TLR) for early evidence planning or a systematic literature review (SLR) and indirect treatment comparison (ITC) for a regional or national health technology assessment (HTA), your evidence must be methodologically sound and communicate product value effectively.
What Sets Us Apart
-

Expert Led
At GIPAM, we go beyond delivering standard evidence synthesis services, with leaders that bring over 18 years of experience driving strategy and content. With senior expert involvement at every step, this ensures:
The right methodology is applied to address the right clinical and economic questions.
The evidence generated is relevant to local market access needs, ensuring product value and payer confidence.
-
Integrated
We provide a connected approach to evidence generation, seamlessly integrating across GIPAM services—helping you connect the dots. For evidence synthesis this means:
Generating a protocol that captures evidence across your simulated PICOs for regional to local market access needs.
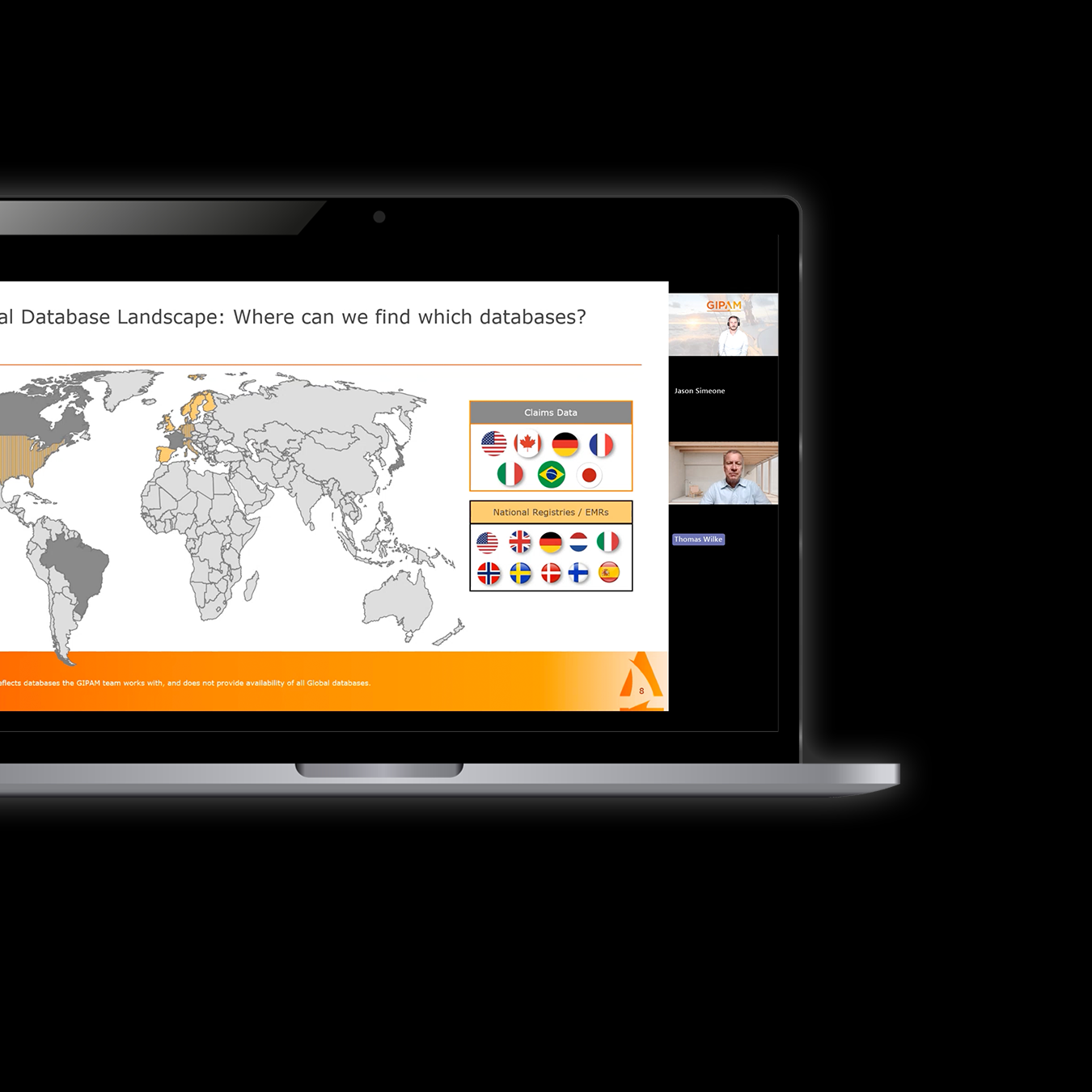
The ability to identify gaps, and potential database sources for real-world evidence.
Expertise to consider the totality of evidence sources from published literature, to real-world studies and synthetic control arms, to inform the most appropriate comparative effectiveness research.
-
Automated
At GIPAM, we leverage proprietary automation tools, including ANCHOR, designed to streamline the evidence synthesis process. ANCHOR eliminates the need for literature review expertise when using automated solutions by providing:
Cochrane-standard automated search strategies
Screening based on clinically relevant PICO criteria
Data extraction designed per indication, collecting data on clinically relevant baseline characteristics and treatment effect modifiers.
Rely on software that is ANCHORed in evidence-based literature review practice and built to each indication.
Our Solutions
What we think
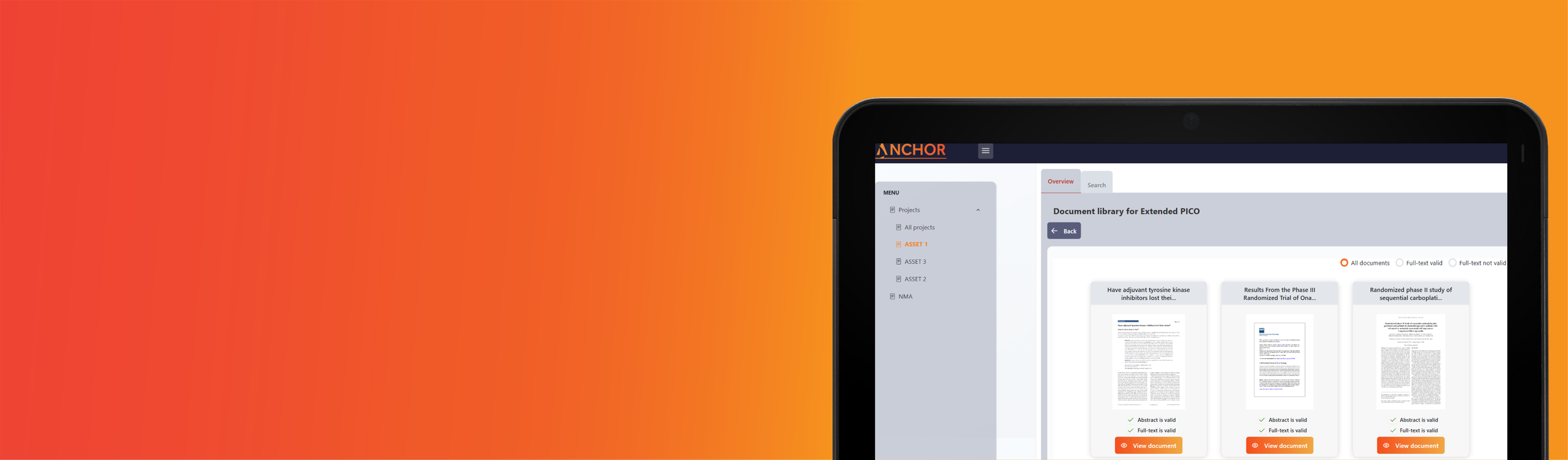
ANCHOR
Literature Reviews: Automated data extraction - data sheets in minutes!